Chemistry in the Modern World: Everything You Need to Know About Air Pollution
MU Mastermind, Domantas (Natural Sciences (Chemistry), University of Cambridge), is interested in a field of research which explores how chemistry can be used to produce and store clean energy to fight global issues of air pollution and man-made global warming. Explore this topical field of chemical research to know more about uses of chemistry in the modern world, and consider the multiplicity of factors involved in combatting climate change.
What causes air pollution?
With booming heavy industry and transport, large amounts of harmful substances escape into the atmosphere, making air pollution a relevant modern problem. Climate change, acid rain and ozone holes are all phenomena linked to air pollution. Luckily, the processes behind all this can be understood with some knowledge in natural sciences. In this article, I will explain various origins and solutions to air pollution, including greenhouse gases, nitrogen and sulphur oxides and chlorofluorocarbons. Finally, I will conclude the challenges to solving air pollution.
Greenhouse gases
You will most definitely have heard of concepts of greenhouse effect and climate change. These are the result of increased emissions of greenhouse gases, for which we would need a brief introduction into how greenhouse gases absorb radiation and how the Earth generates this radiation. To start with, the surface of the Earth is constantly receiving heat by absorbing light from the sun, which is why temperatures are warmer here on Earth than on planets further away from the sun. In addition, the Earth loses heat by Blackbody radiation: warm bodies radiate in the infrared (IR) spectrum, while above around 600°C objects start to emit visible light in addition to infrared.
Now on to the greenhouse gases. Generally, chemical bonds in molecules vibrate, but they can only possess certain amounts of vibrational energy, visualised as this “ladder” of allowed energy levels. The molecule can “ascend” from one energy level to another by absorbing a photon whose energy matches the energy separation of the two levels. These vibrational level separations happen to be of the infrared radiation energy, therefore any atmospheric gas that can absorb IR light reduces the amount of heat that is leaving the Earth. Because the average temperature is determined by the balance of heat entering the Earth’s atmosphere and the heat leaving it, an increase in greenhouse gas concentration will cause the atmosphere to slowly heat up. Common greenhouse gases are carbon dioxide (CO2) and methane (CH4), which are the main contributors to climate warming. Carbon dioxide plays an extremely important role in life: it is consumed by plants, algae, and some bacteria for photosynthesis, and hence is the source of carbon for any food chain. Due to its prevalence in life, CO2 may not sound as a “bad thing”. However, the CO2 concentration in air has increased from the pre-industrial average 280 ppm (parts per million) to a current 420 ppm. This deviation from the balance has already caused a rise in temperatures and more extreme weather worldwide.
Methane, CH4, is also a greenhouse gas and a major constituent of natural gas. It is naturally released into the atmosphere because of anaerobic decomposition – when dead biomatter decomposes in the absence of oxygen. Anthropogenic sources of methane account for 60% of all methane pollution and include fossil fuel extraction, coal mining, landfills, and agriculture – primarily cattle farms and rice fields. Even though the concentration of methane in the atmosphere (1.8 ppm) is much lower than that of CO2, methane still contributes a significant amount to the greenhouse effect, due to the fact that a CH4 molecule can absorb much more heat than CO2 can.
Nitrogen and sulphur oxides
Nitrogen oxides (such as N2O, NO and NO2) and sulphur oxides (mainly SO2) are significant pollutants that cause of acid rain and respiratory health problems. These gases occur naturally by decomposition of biomatter and volcanic eruptions. Man-made nitrogen and sulphur oxide pollution originates mostly from transport and industry. For example, nitrogen dioxide is generated inside car engines as atmospheric nitrogen and oxygen react at very high temperatures, while sulphur dioxide emitted by burning coal that naturally contains sulphur. Nitrogen oxide pollution is a significant problem in large cities, especially those with intense industry and traffic.
Photochemical Smog
Nitrogen oxides and volatile organic compounds, such as fuel vapours, can react under intense sunlight to produce what is called photochemical smog. The UV light in the sunlight can break chemical bonds in molecules, producing highly reactive free radicals that then react with the hydrocarbons, nitrogen oxides and atmospheric oxygen. One of the reaction products is ozone, which is harmful to the respiratory system and may increase respiratory symptoms in people with asthma. Los Angeles, the largest US west coast city, has one of the largest numbers of personal automobiles per household in the US. Photochemical smog is often visible as a brown-coloured fog rising a few hundred metres above the ground.
A good solution to urban air pollution is more use of public and electric transport. While electric cars reduce air pollution in the city, they do not necessarily reduce overall greenhouse gas emissions as the power may still come from fossil fuels.
Acid rain
In humid air, nitrogen dioxide can react with water vapour to form nitric acid (HNO3) and nitric oxide (NO) which can then be oxidised to produce more NO2. Meanwhile, sulphur dioxide is slowly oxidised by oxygen in the air, creating sulphur trioxide SO3, which in turn reacts with water to produce sulphuric acid (H2SO4). Nitric and sulphuric acids are both strong acids. That means that, in water, they fully dissociate into H+ and the corresponding anion: NO3- or HSO4-, respectively, lowering the pH of water to as low as 2. Acidic rainwater is detrimental to buildings and statues made of acid-soluble materials such as limestone and concrete. Many plants and aquatic animals cannot tolerate acidic water, hence acid rain results in the death of fish and forests. Regions with heavy industry are badly affected by acid rain. In the 70s and 80s a European region encompassing parts of present-day Czechia, Germany and Poland suffered huge environmental damages due to acid rain caused by coal and metallurgic industries. The severity of the damage granted the region a name “The Black Triangle”. Like many other affected areas in Europe and North America, this area is recovering after the extent of heavy industry decreased. However, acid rain is becoming a problem in highly populated regions of rapidly developing countries such as China and India.
Chlorofluorocarbons (CFCs)
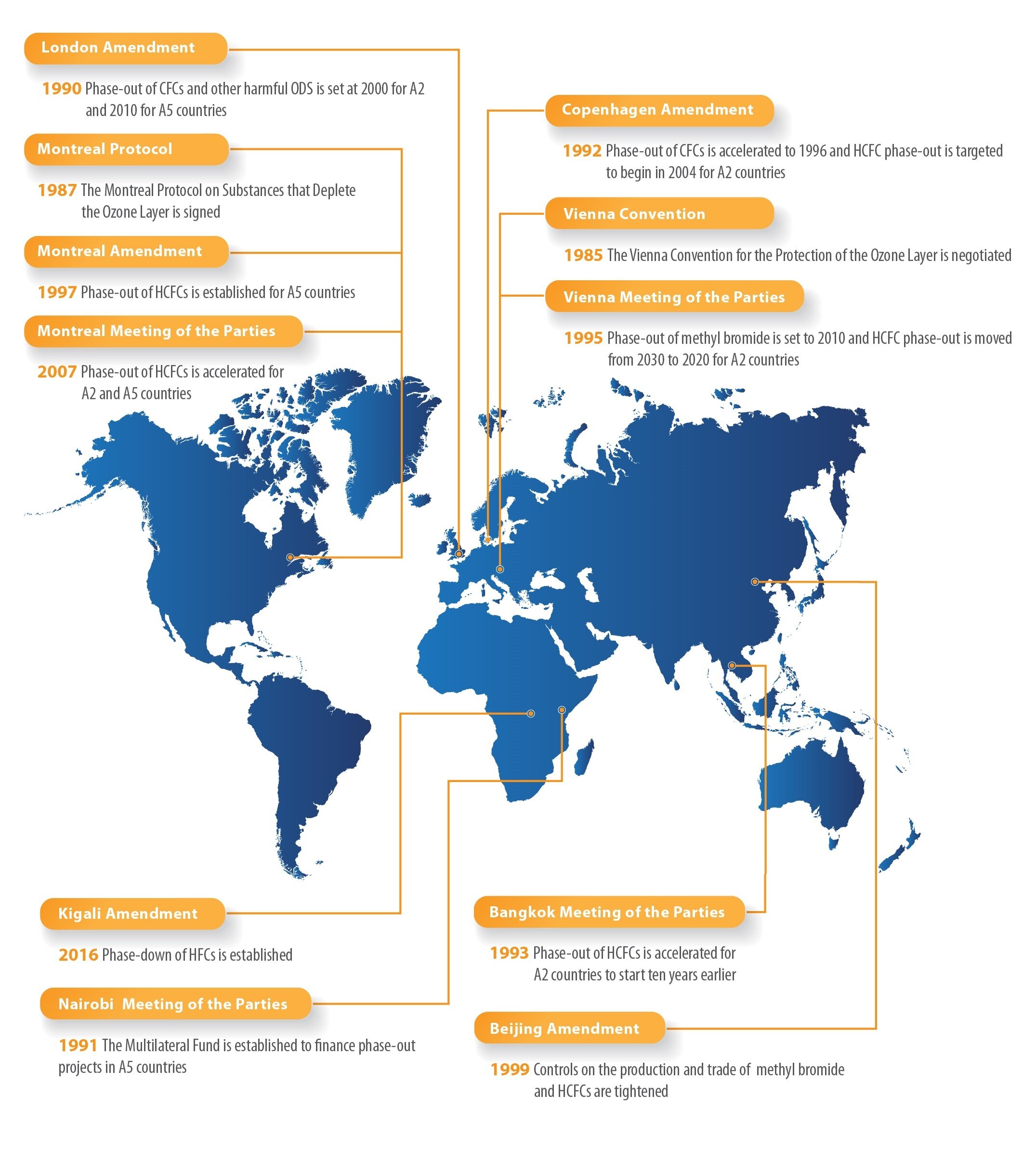
Chlorofluorocarbons are hydrocarbons with some of their hydrogen atoms exchanged for chlorine and fluorine atoms. These compounds are generally non-flammable, non-toxic volatile gases, and liquids with boiling points suitable for use for refrigeration purposes. They were used extensively for this purpose before being phased out following the 1987 Montreal Protocol. The reason for the ban on CFCs was the depletion of the ozone layer. The process that causes stratospheric ozone depletion, like smog case, is also a radical reaction: UV light can break off a chlorine atom of the CFC, making the chlorine very reactive. It can then react with ozone and destroy it. By 1980s, large regions depleted of ozone formed around the world, most significantly in the polar latitudes. To prevent further ozone depletion, CFCs have been replaced with non-harmful hydrofluorocarbons. Since then, stratospheric ozone has been steadily replenishing and is expected to reach the pre-1980 levels by around 2075.
https://www.epa.gov/ozone-layer-protection/international-actions-montreal-protocol-substances-deplete-ozone-layer - The international treaty, The Montreal Protocol on Substances that Deplete the Ozone Layer (Montreal Protocol), gradually aims to eliminate the production and consumption of ozone depleting substances to limit their damage to the earth’s ozone layer. Signed by 197 countries – the first treaty in the history of the United Nations to achieve universal ratification, it is considered by many the most successful environmental global action.
How COVID-19 cut air pollution in 2020
The 2020 coronavirus pandemic resulted in strict measures to slow down the spread of the virus, including restrictions on travel and work in sectors ranging from services to industry. Since many people had to work from home, commuter traffic decreases significantly, drastically cutting pollutant concentrations. We can now learn how to transform strict lockdown measures into economically sustainable home office culture to tackle bad air quality.
Some aspects and issues of reducing air pollution
In addressing the issue of air pollution, the scope of measures is important. Some types of air pollution, such as smog and acid rain, can be dealt with locally. Meanwhile, greenhouse-gas caused climate change and ozone depletion are global problems that require measures to be taken by all parties contributing to the environmental issue.
Cutting air pollution is, on one hand, a technological challenge. Current technology has progressed a long way to provide tools to live without air pollution, such as wind turbines and solar cells. However, clean technology still needs to be developed and optimised. For example, electric cars are not yet very prevalent partly due to expensive batteries. On the other hand, reducing pollution is also a political challenge. It involves making decisions on making big investments into new infrastructure. In addition, pressuring large companies to reduce their pollution is not always straightforward.
Finally, intense air pollution has been most pronounced in developing countries that need large amounts of cheap energy, often from fossil fuels. At the same time, only more affluent countries can afford to switch to more expensive but cleaner energy sources.
Understanding the chemistry of air pollution is key to grasping how to combat it. If you are interested in finding solutions to climate change, why not research potential solutions? A diesel phase-out by 2025, electric vehicles, local, regional & global action, renewable fuels, circular economy of solid wastes…brainstorm efficient solutions, and work out how you would like to contribute to change.
Are you considering a Natural Sciences degree?
Check out our tuition side, U2’s blog on applying for Biological Natural Sciences, and contact us if you are interested in exploring beyond the curriculum with one of our Cambridge Natural Scientists in preparation for application.
Would you like to stay updated on new Sustainability/ STEM-related content?
Check out our free Curious Minds digest - we send out individualised resources to students interested in staying updated on the latest developments. We suggest related essay competitions, give reading recommendations, and notify you of upcoming events in your field of interest.